What Is the Molar Mass of Iron Sulfate
Chemistry questions and answers. Iron III sulfateA few things to consider when finding the molar mass for Fe2SO43- make sure you.
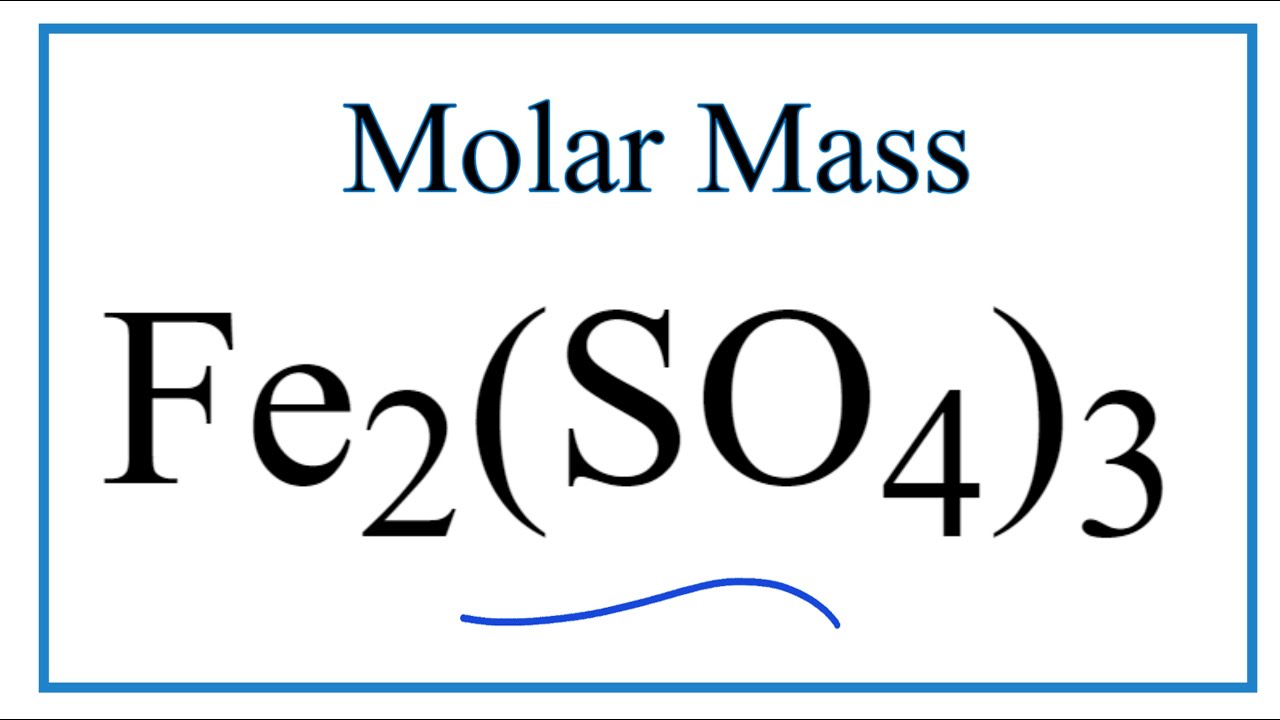
Molar Mass Molecular Weight Of Fe2 So4 3 Iron Iii Sulfate Youtube
See also Is yusuke a demon.

. What is the empirical formula for this compound. FeSO47H2O is a light green crystal at room temperature. Enter your answer to two decimal places.
What Colour is iron sulfate. FeSO 4 H 2 O. Solution for What is the molar mass in gmol¹ of anhydrous ironIII sulfate to the nearest whole number.
1 mole is equal to 1 moles Fe2SO43 or 3998778 grams. Iron II Sulfate Heptahydrate. The mono- penta- e hexahydrate salts are also largely used.
Calculate the molar mass of iron II sulfate. Iron II sulfateA few things to consider when finding the molar mass for FeSO4- make sure you have the. Convert grams Iron III Sulfate to moles or moles.
4 rows Iron Sulfate molecular weight. Explanation of how to find the molar mass of FeSO4. 5 6 7 8 B C N O 1081 1201 141 1600 13 14 15.
Iron sulfate is a white orthorhombic hygroscopic crystalline system. None of the above 3. If the formula mass of ironII sulfate FeSO 4 is 1519 amu what is the molar mass of ironII sulfate Molar mass 1519 gmol Mass of one mole is 1519g.
What is the molar mass of fe2 so3 3. а СНО and 116 CHO CHO CHO Not enough information to solve 4. Molar mass of FeSO 4 IronII Sulfate is 1519076 gmol.
Iron Fe Hydrogen H Oxygen O Sulfur S Molecular weight. The iron II sulfate chemical formula is FeSO 4The molar mass is 15191 gmol. Iron sulfate monohydrate is a yellow to white monoclinic crystalline system.
A 104 B 248 C 336 D 400. What is the mass in gram of 325 mol of Fe2 SO4 3 You can view more details on each measurement unit. Question 1 What is the molar mass of IronIII sulfate Fe2SO4.
2 196 g Fe 2 SO 4 3 contains how many grams of sulfur. Fe 2 SO 4 3. 4 rows Molar mass of Fe2 SO43 3998778 gmol.
Acompound that contains only carbon hydrogen and oxygen is 4864 hy mass. Convert grams Iron. This salt is found in a broad range of hydrated salts with the formula FeSO 4xH 2 O while the most widely used is the heptahydrate iron II sulfate FeSO 47H 2 O with the molar mass of 27802 gmol.
Answer39988 gmolExplanationIronIII sulfate is the chemical compound with the formula Fe₂SO₄₃. 3 How many molecules are in 280 g of isopropyl alcohol C 3 H 8 O. The mass in grams of 140 mol of anhydrous ironIII sulfate is 559832.
Molecular weight of Fe2SO43 or grams This compound is also known as IronIII SulfateThe SI base unit for amount of substance is the mole. Mass percentage of the elements in the composition. 1 mole of ferric sulfate contains 2 moles of iron and 12 moles of oxygen atoms and three moles of sulfate ions.
1 What is the molar mass of iron III sulfate. Explanation of how to find the molar mass of Fe2SO43. How is Fe 3 formed.
CAS Registry Number CAS RN. What is the mass in grams of 140 mol of iron III sulfate. Usually yellow it is a salt and soluble in wa.
Iron Fe Oxygen O Sulfur S Molecular weight. Molar mass of FeSO4 1519076 gmol.

Molar Mass Molecular Weight Of Feso4 7h2o Iron Ii Sulfate Heptahydrate Youtube

Molar Mass Molecular Weight Of Feso4 Iron Ii Sulfate Youtube

Molar Mass Molecular Weight Of Feso4 Iron Ii Sulfate Youtube
0 Response to "What Is the Molar Mass of Iron Sulfate"
Post a Comment